Keeping pace with contraception: a practical refresher for pharmacists
The rapidly expanding scope of pharmacists continues to provide new opportunities for pharmacy teams to deliver accessible and timely care for Canadians. One area where our role is quickly changing is in the prescribing of contraceptives, where pharmacy scope has gradually expanded to several provinces, including BC, AB, SK, QC, NB, and NS. Most recently, British Columbia has proposed regulations for pharmacists to prescribe contraception along with medications for select minor ailments- their Minor Ailments and Contraceptive Service is in development and set to roll out in June 2023. In the meantime, starting April 1, 2023, most contraceptive products (combined oral contraceptive pills, intrauterine device, implant, injection, emergency contraceptive pill) have been listed in BC PharmaCare providing full or partial coverage for all BC residents.
There is a stark need for these services; up to half of all pregnancies are unintended, often disproportionately affecting vulnerable populations such as younger individuals, Indigenous populations, new immigrants, patients living in rural communities, and patients of lower socioeconomic class.1,2 As over one million women in Canada face barriers to obtaining contraception, expanding pharmacy scope (along with coverage) can facilitate timely and equitable access to these products.3
Regardless of the scope in your province, providing contraception is an essential component of pharmacy practice. In this refresher on contraception, we will review key considerations to help optimize contraceptive care for your patients.
NAVIGATING THROUGH THE OPTIONS
Options for contraception include the implant, intrauterine devices (IUDs), injection, ring, patch, and pill. Within these choices, the first consideration may be to determine if patients would like a long-term solution or a more flexible method.
Long-acting birth control options:
- The progestin implant, Nexplanon®, offers over 99% effectiveness for up to three continuous years. Like other hormonal long-term birth control options, common side effects include amenorrhea, headache, spotting, and breast soreness. As it is inserted subdermally in the arm, a scar may develop at the site of insertion.4
- Hormonal Intrauterine Devices (IUD) have a comparable effectiveness to Nexplanon®. Approved options (Mirena® and Kyleena®) are effective for up to five years (note: you may encounter questions from patients on this as the FDA in the United States has extended the indication for Mirena to eight years). Mirena® is also indicated to treat heavy periods (menorrhagia).5-10
- The Copper IUD also offers over 99% effectiveness, however compared to hormonal alternatives it can lead to heavier periods. A unique feature is that it does not require any backup contraception when started (7 days of backup is generally recommended for most other options) and can also serve as an emergency contraceptive when inserted within seven days of unprotected intercourse. Copper IUDs are more affordable and available for various durations (3, 5, 10 years), they may however, be associated with side effects such as dyspareunia (pain during sex), dysmenorrhea, and menorrhagia.11
- The injection, Depo-Provera®, administered every 12-13 weeks, is slightly less effective at around 94%. As patients may experience a delay in return to fertility, those who are planning a pregnancy in the next 1-2 years may be counseled to avoid this method. Depo-Provera has been associated with weight gain and a loss in bone mineral density, therefore it is only recommended to be used for up to two years.12
Individuals who desire shorter-term contraception and greater cycle control may find the following options (all ~91% efficacy) more preferable.13,14
- Combined oral contraceptives (COCs) are the most common hormonal method. While COCs provide greater cycle control, they may also leave room for user error/adherence concerns due to the required daily dosing.
- The mini-pill (progestin only) provides an oral option to patients who are contraindicated/intolerant to estrogen. As the mini-pill works by thickening cervical mucus and thinning uterine lining, backup contraception is necessary if the dose is more than 3 hours late.15
- The contraceptive patch (Evra®), operates on a weekly adherence schedule which may be more convenient for some patients. Patch specific side effects include skin irritation and breast soreness.16
- The contraceptive vaginal ring (NuvaRing®) is used for a cycle of three weeks, plus one week off. The most common side effects specific to the ring are vaginal irritation/discharge or expulsion of the ring.17
Please note, contraindications for combined hormonal contraceptives (COCs, Evra, Nuvaring) include but are not limited to patients with breast cancer, migraines with aura, uncontrolled hypertension, past or current cardiovascular disease or thrombosis, liver disease, smokers over 35 years old, complicated diabetes, etc.18 For patient-friendly guidance on missed dosing, check out this tool: https://www.sexandu.ca/sos/sos.php.
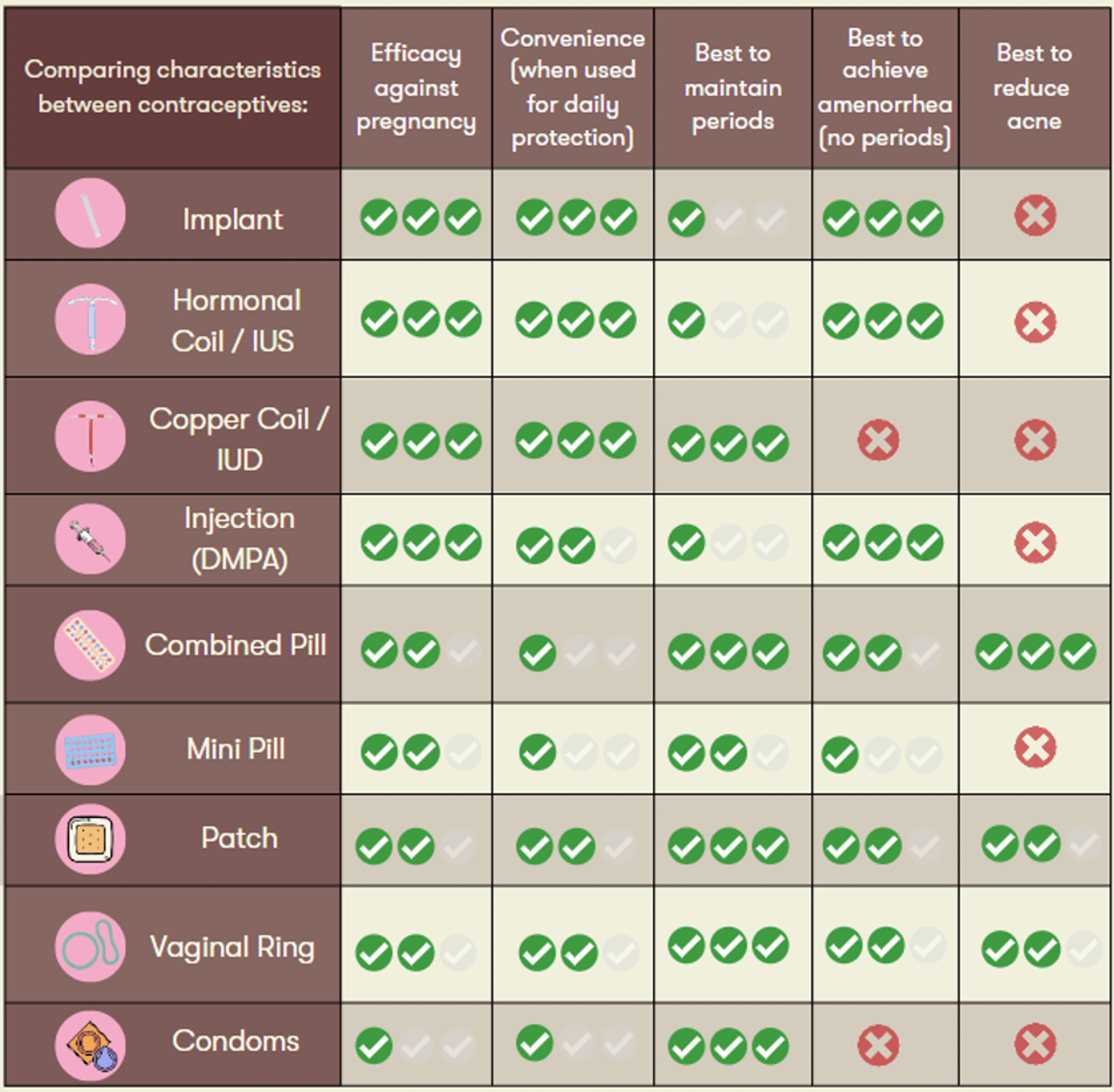
A personalized approach is important to support patients along their desired reproductive health goals. As such, the chart below can serve as a useful tool to guide your shared decision making.
For more guidance on switching between birth control options, visit this link
MORE THAN CONCEPTION CONTROL
While the most common indication for contraception is to prevent pregnancy, more than half of patients who take COCs use them for their additional effects.
Most commonly, COCs can provide patients greater control over their menstrual cycles (e.g, lighter, more regular periods, skipping periods). Other indications beyond contraception include use for endometriosis, acne, hirsutism, dysmenorrhea, and premenstrual syndrome.18 The hormone content and balance between estrogen and progesterone within COCs will impact both therapeutic indications and side effects. For instance, a lower estrogen dose may be required in patients who experience nausea, breast tenderness, or general headaches. On the other hand, patients who experience weight gain, bloating, acne or mood changes may benefit from a reduction or change in the progestin content of their COCs. When it comes to breakthrough bleeding, a higher estrogen content may improve spotting within the first half of the cycle whereas higher progestin can help if spotting occurs in the second half of the patient’s cycle. Patients should be counselled that it can take three months for side effects (such as nausea, bloating, breast tenderness) to improve as they adjust to a new birth control method, even when switching between COC options.
COCs are also available as monophasic or multiphasic regimens, categorized based on their progesterone content. Monophasic COCs contain a consistent level of progesterone within their active pills, whereas multiphasic options aim to replicate the body’s natural hormone fluctuations by containing varying progesterone levels. Tri-Jordyna® (generic alternative to discontinued Tri-Cyclen®), for instance, has the same amount of ethinyl estradiol (0.035 mg) for its 21 days of active pills, while the amount of norgestimate (a third-generation progestin) varies from 0.18 mg in the first week, 0.215 mg in the second week, and 0.25 mg in the third week. Although comparative data is limited, monophasic options are generally first-line for patients starting COCs, however patients who experience persistent side effects (such as amenorrhea or breakthrough bleeding) may find a switch to triphasic products beneficial.19
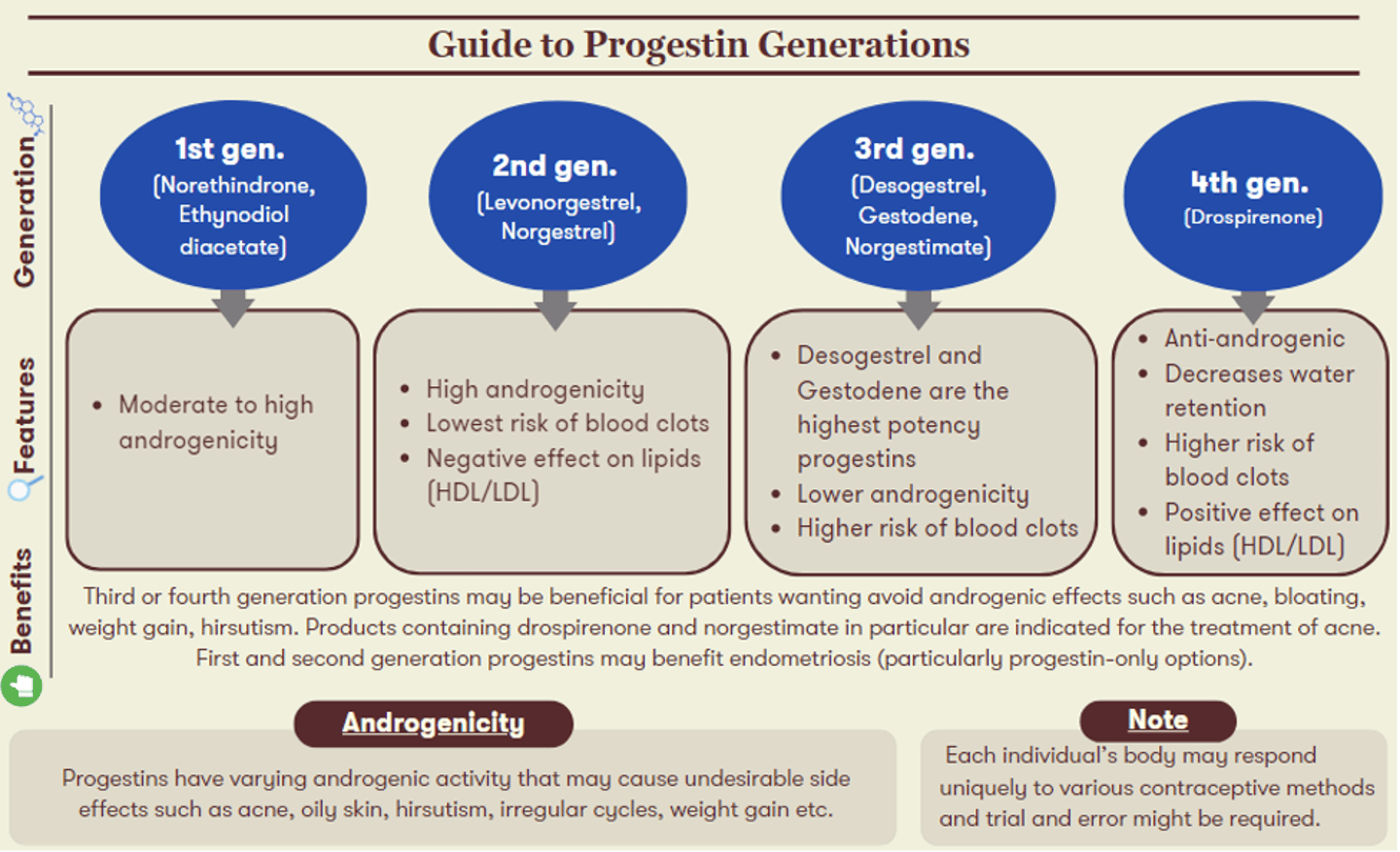
It is important to keep in mind that everyone responds uniquely to various contraceptive methods and trial and error might be required. The generation of progestin in a COC will also influence its associated uses and side effects. The figure below highlights characteristics of each progestin generation, alongside recommendations for which patient candidates may experience the greatest benefits from their use.
OPTIMIZING THERAPY
When recommending or switching birth control methods, it is crucial to consider factors unique to the patient. Common factors include managing or reducing unwanted side effects, such as weight gain, irregular bleeding, acne, or hirsutism, as well as personal preferences relating to efficacy, adherence, and comfort. It is important to thoroughly evaluate the patient’s intended goals and overall satisfaction as poor adherence can lead to unintended pregnancies.
It is also important to remember that although hormonal contraception is more effective (with proper use) for conception control than barrier methods, condoms are the only method that will protect patients against sexually transmitted infections.
There are many choices when considering contraception and it can be overwhelming for patients to select the best option. As pharmacists, we play a critical and growing role in helping patients navigate the available alternatives to make informed decisions about their reproductive health. By taking into consideration the characteristics of therapeutic options along with the patient's medical history, lifestyle choices, and personal goals, we can guide them towards a contraception option that best fits their unique needs.
Molly Yang is the Director of Pharmacy Innovation & Professional Affairs at Wholehealth Pharmacy Partners. This article was developed with support from Glenmark Pharmaceuticals Canada. All opinions contained belong to the authors and do not necessarily reflect the opinions of the sponsor.
- Oulman, E., Kim, T. H., Yunis, K., & Tamim, H. (2015). Prevalence and predictors of unintended pregnancy among women: an analysis of the Canadian Maternity Experiences Survey. BMC pregnancy and childbirth. Retrieved March 29, 2023, from https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-015-0663-4
- Hulme, J., Dunn, S., Guilbert, E., Soon, J., & Norman, W. (2015). Barriers and facilitators to family planning access in Canada. Healthcare Policy. Retrieved March 29, 2023, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4748342/
- Contraception Infographic - Canadian Pharmacists Association. (n.d.). Retrieved March 29, 2023, from https://www.pharmacists.ca/cpha-ca/assets/File/cpha-on-the-issues/Contraception-Infographic.pdf
- Canadian Drug Product Database. Product Information Nexplanon®. (2019) Government of Canada. Available from: https://pdf.hres.ca/dpd_pm/00060728.PDF
- Guida, M., Farris, M., Aquino, C. I., Rosato, E., Cipullo, L. M. A., & Bastianelli, C. (2019). NEXPLANON subdermal implant: Assessment of sexual profile, metabolism, and bleeding in a cohort of Italian women. BioMed research international. Retrieved March 29, 2023, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6374865/
- Moray, K. V., Chaurasia, H., Sachin, O., & Joshi, B. (2021). A systematic review on clinical effectiveness, side-effect profile and meta-analysis on continuation rate of Etonogestrel contraceptive implant - reproductive health. BioMed Central. Retrieved March 29, 2023, from https://reproductive-health-journal.biomedcentral.com/articles/10.1186/s12978-020-01054-y
- Bofill Rodriguez, M., Lethaby, A., & Jordan, V. (2020). Progestogen-releasing intrauterine systems for heavy menstrual bleeding. The Cochrane database of systematic reviews. Retrieved March 29, 2023, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7388184/
- V;, B. R. M. L. A. J. (n.d.). Progestogen-releasing intrauterine systems for heavy menstrual bleeding. The Cochrane database of systematic reviews. Retrieved March 29, 2023, from https://pubmed.ncbi.nlm.nih.gov/32529637/
- Canadian Drug Product Database. Mirena®. (2021) Government of Canada. Available from: https://www.bayer.com/sites/default/files/mirena-pm-en.pdf
- Canadian Drug Product Database. Kyleena®. (2021) Government of Canada. Available from: https://www.bayer.com/sites/default/files/2020-11/kyleena-pm-en.pdf
- Black, A., Guilbert, E., Costescu, D., Dunn, S., Fisher, W., Kives, S., ... & Todd, N. (2015). Canadian contraception consensus (part 1 of 4). Journal of Obstetrics and Gynaecology Canada. Retrieved March 29, 2023, from https://www.jogc.com/article/S1701-2163(16)30260-2/pdf
- Canadian Drug Product Database. Depo-Provera®. (2022) Government of Canada. Available from: https://www.pfizer.ca/files/DEPO-PROVERA_PM_EN.pdf
- Trussell, J., & Guthrie, K. (2007). Choosing a contraceptive: efficacy, safety, and personal considerations. Contraceptive technology, 19. Retrieved March 29, 2023, from https://www.researchgate.net/publication/285756856_Choosing_a_contraceptive_Efficacy_safety_and_personal_considerations
- Abramowicz M (2010). Choice of contraceptives. Treatment Guidelines From The Medical Letter. Retrieved March 29, 2023, from https://secure.medicalletter.org/TG-article-100a
- Canadian Drug Product Database. Movisse®. (2016) Government of Canada. Available from: https://pdf.hres.ca/dpd_pm/00034254.PDF
- Canadian Drug Product Database. Evra®. (2018) Government of Canada. Available from: https://pdf.hres.ca/dpd_pm/00046284.PDF
- Canadian Drug Product Database. NuvaRing®. (2018) Government of Canada. Available from: https://pdf.hres.ca/dpd_pm/00046305.PDF
- Black, A., Francoeur, D., Rowe, T., Collins, J., Miller, D., Brown, T., ... & Henneberg, E. (2004). Canadian contraception consensus. Journal of obstetrics and gynaecology Canada: JOGC= Journal d'obstetrique et gynecologie du Canada: JOGC, 26(4), 347-87. Chicago. Available from: https://www.jogc.com/article/S1701-2163(16)30260-2/pdf
- Van Vliet, H. A., Grimes, D. A., Lopez, L. M., Schulz, K. F., & Helmerhorst, F. M. (2011). Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database of Systematic Reviews, (11). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7154342/